Activated Carbon for Iron Removal
Industrialization promotes economic and social development, but growing industrial facilities intensify pollutant emissions, affecting the entire ecosystem. Water pollution is one of the harrowing effects of industrialization.
Data shows that nearly 60% of surface water and 50% of drinking water contain iron ions. Due to mining activities, treatment plants and other activities, the iron content in water sources has increased significantly in some areas. Excessive iron accumulation may cause serious health problems.
In this study, activated carbon adsorption technology was used to treat iron in water. During the experiment, the feasibility of removing iron ions by batch and fixed bed adsorption was studied.
Introduction of iron
The 26th element iron in the periodic table is an important element. It covers about 5% of the Earth’s crust and is the second most abundant metal, after oxygen, silicon and aluminum among the elements.
Elemental iron is a transition metal. The basic oxidation states are +2 (ferrous) and +3 (ferric), although there are also +4 (ferrous) and +6 (ferrate) oxidation states. Iron compounds in the +2 state are labeled ferrous and consist of light green Fe ions, while iron compounds in the +3 state are labeled ferric and contain Fe complex ions, which turn from yellow to orange and eventually brown, depends on the degree of hydrolysis.
In oxygenated water, Fe2+ ions are oxidized to Fe3+ ions. The oxidation rate depends mainly on the H+ ion concentration and the solution temperature. Ferrous ions generally retain a higher amount of iron in solution than ferric ions, and ferrous elements tend to have higher solubility compared to elemental iron.
Adsorption for iron removal
Iron is highly toxic and, therefore, it is necessary to control this pollutant at emission levels. There are many technologies available for iron removal from drinking water and municipal waste, such as ion exchange and water softening, supercritical fluid extraction, aerated oxidation, microfiltration/ultrafiltration, aerated particulate filters, and bioremediation.
The main disadvantage of these technologies is that they are either expensive or cannot be used without electricity. Adsorption is the adhesion of an adsorbed substance (adsorbate) to a solid surface. Adsorbates exist in the fluid phase as solutes dissolved in liquids or gases. It is favored over other treatments due to its ease of use, simple design, high capacity, low cost, low by-product generation, and high therapeutic efficacy.
This is a surface phenomenon that requires pollutants in the wastewater to accumulate on the surface of the adsorbent. This deposition of metal ions on the adsorbent occurs at the interface of the adsorbent, resulting in a two-dimensional structure. This depends on the properties of the adsorbent, such as surface charge, surface area, and surface functionality.
Adsorption is also superior to other removal processes because of its high efficiency and the possibility to regenerate the adsorbent and recover the adsorbate. One of the ways that these difficulties can be overcome is the adsorption of iron by various adsorbents. Due to its multifunctional behavior, activated carbon has been identified to be highly active in wastewater treatment.
Activated carbon structure
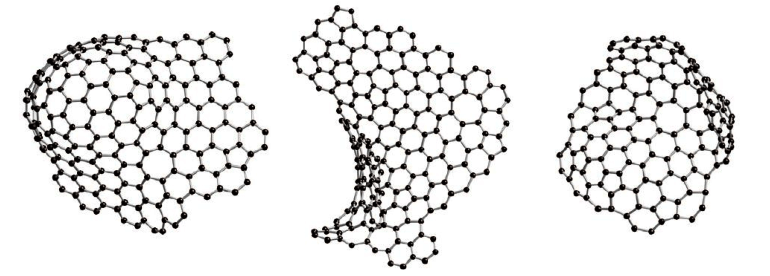
The exact atomic configuration of activated carbon is unknown, despite its commercial importance in water and air purification. Configuration of carbon is prepared by pyrolysis.These carbons can be classified into two distinct groups, graphitized and non-graphitized. Derived carbon of activated carbon is non-graphitizable, which means it can transform into crystalline graphite even at very high temperatures (≥3000℃).
Neutron diffraction studies have shown that non-graphitizable carbons have a framework similar to fullerenes, as shown in Figure 1. But there has been not able to provide any clear evidence that the atoms are bonded in the form of pentagonal or hexagonal rings.
Activated carbon pores
In addition to the crystallization and chemical configuration of activated carbon, its porous structure also plays a crucial role in various applications. The absorption capacity of activated carbon is highly related to surface area, pore volume and pore size distribution.
It mainly depends on the nature and chemical treatment of raw materials. The porous structure of activated carbon is produced by eliminating non-carbon substances in the raw material during carbonization, which produces a fixed carbon block with a basic pore structure and further developed during the activation process.
The activation process enlarges the diameter of the pores created during carbonization and at the same time creates some new pores, resulting in a well-developed porous structure. The pores in activated carbon are distributed in various sizes and shapes. The pore size distribution of the resulting activated carbon is mainly affected by the degree of impregnation. Activation and carbonization conditions are the most critical parameters affecting the porosity type of the resulting activated carbon.
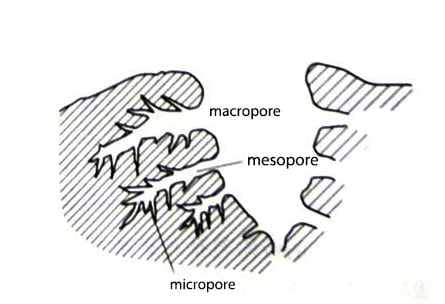
Activated carbons are classified by pore size as follows: macropores (>50nm), mesopores (2–5nm), and micropores (<2nm). Micropores can be further subdivided into ultramicropores (<0.5nm) and ultramicropores (1-2nm). The mesopores serve as pathways for adsorbate molecules to pass through the microporous network. Macropores serve no purpose, but they help guide metal ions into the mesopores and microporous surfaces. The characteristic pore size distribution of activated carbon is shown in Fig. 2.
Working condition of activated carbon for iron
The adsorption process is largely dependent on various constraints such as pH, sorbent dosage, initial concentration, contact time, and temperature. The effect of these parameters on the adsorption of iron by activated carbon was studied in detail to determine the optimal conditions.
Activated carbon was used to exclude Fe(II) ions from the aqueous phase in several pH ranges from 2 to 6. It is found that at pH<3, the removal efficiency was the lowest. As the pH value of the solution increased, the removal percentage increased significantly, and the maximum removal rate was reached at pH5.0.
The amount of iron adsorbed on activated carbon increases with the contact time. Due to the high driving force between the adsorbent and metal ions in solution, the adsorption rate was fast in the first 90 min.
Adsorption sites accessible at the beginning, slow down and reach equilibrium in around 150 min. Extending the run time to 6 h had no effect on the residual concentration of metal ions, indicating that equilibrium was reached at 150 min, beyond which the accumulation of iron ions made it difficult to diffuse into the sorbent. Diffusion resistance increases with enhanced micropore filling, which contradicts the effect of contact time.
The removal of metal ions is a highly concentration-dependent process that can be recognized as an effect of mass transport. Adsorption is regulated by the monolayer coverage of molecules on the activated carbon sorbent boundary.
Therefore, the adsorption process is initially very rapid. At higher concentrations, the vacancies present on the carbon surface will be surrounded by more adsorbates. Therefore, the metal ions were not only adsorbed by the monolayer on the peripheral surface of the activated carbon adsorbent, but also diffused into the interior of the activated carbon particles.
Conclusion
Activated carbon adsorbs to eliminate the impact of iron on the environment. Although iron is an important mineral for humans, if the iron content in water exceeds a certain level, it will make the water undrinkable, resulting in various health disorders. Providing people with safe drinking water is a universal human need. Therefore, efficient and affordable technologies are essential to use activated carbon for water treatment to remove iron from water to ensure safe drinking water.