Activated carbon is formed with carbon as the main component. After activation, activated carbon has a random or amorphous structure consisting of graphite-like microcrystals and hydrocarbons. The structure can have the following characteristics.
Porous structure:
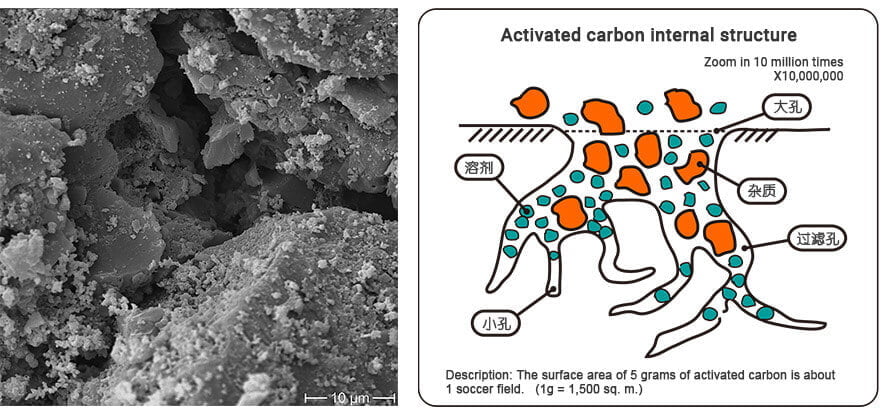
Activated carbon consists of a complex network of pores varying in size, shape and distribution. The porous structure allows for a large surface area, ranging from 500 to 1500 m2 /g or more, depending on the activation process and the raw material used. This high surface area is essential for the adsorption of contaminants.
Pore size distribution
The pores in activated carbon can be divided into three categories according to their size.
- Micropores: pores less than 2nm in diameter. Micropores contribute significantly to the total surface area and are responsible for the adsorption of small molecules. Coconut shell h activated carbon has well-developed micropores.
- Mesopores: Pores between 2 nm and 50 nm in diameter. Mesopores contribute to the transport of adsorbates in and out of the micropores and allow the adsorption of larger molecules. Activated carbon from coal has well-developed mesopores and micropores.
- Macropores: Pores with a diameter greater than 50 nm. Macropores act as the main pathway for the diffusion of macromolecules and facilitate the entry of adsorbates into the internal pore structure. Wood activated carbon is predominantly macroporous.
Carbon lattice
The carbon atoms in activated carbon are arranged in a lattice structure, mainly consisting of hexagonal rings, similar to graphite. However, the carbon lattice in activated carbon is not as well ordered as graphite and has many defects, such as vacancies, dislocations and amorphous carbon regions. These structural defects provide additional sites for adsorption and contribute to the overall adsorption capacity of the material.
Surface chemistry
The surface of activated carbon can contain various functional groups such as hydroxyl, carbonyl and carboxyl groups, which arise as a result of activation processes or the presence of residual organic or inorganic materials. These functional groups can interact with the adsorbate through a variety of mechanisms such as hydrogen bonding, ion exchange and complexation, enhancing the adsorption capacity of the activated carbon.
The structural characteristics of activated carbon make it a highly effective adsorbent for a wide range of pollutants and applications. We can select the right activated carbon for the specific application.